20
Aug

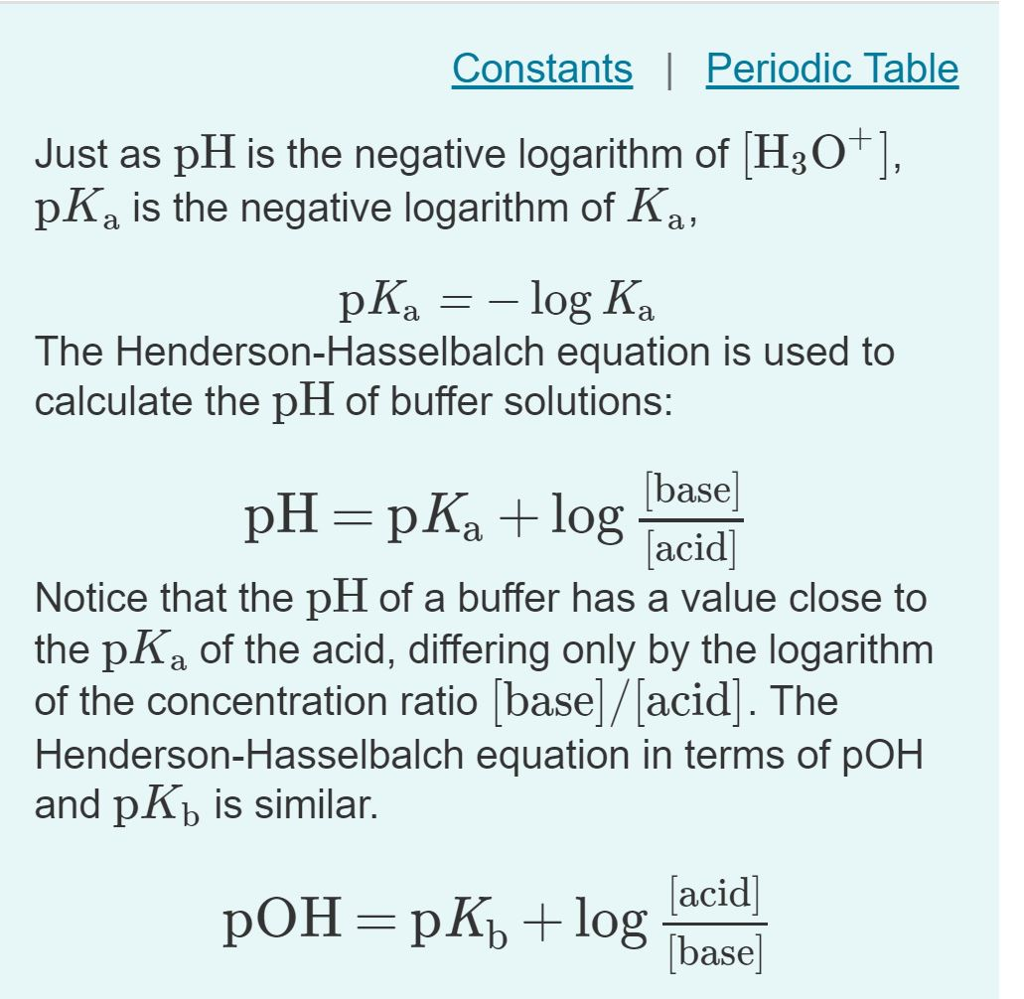
Going back to HCl Acetic Acid. At half the equivalence point. At half the equivalence point pH pKa -log Ka.
Has a pKa of around 157. If you already know the pKa value for an acid and you need the Ka value you find it by taking the antilog. Going back to HCl Acetic Acid. Ini karena nilai pKa yang lebih tinggi menunjukkan bahwa Ka rendah. The stronger an acid the greater the ionization the lower the pKa and the lower the pH the compound will produce in solution. Lets talk about pKas.
HCl Ka 1107.
Previous post
Plaques electriques pour kitchenette